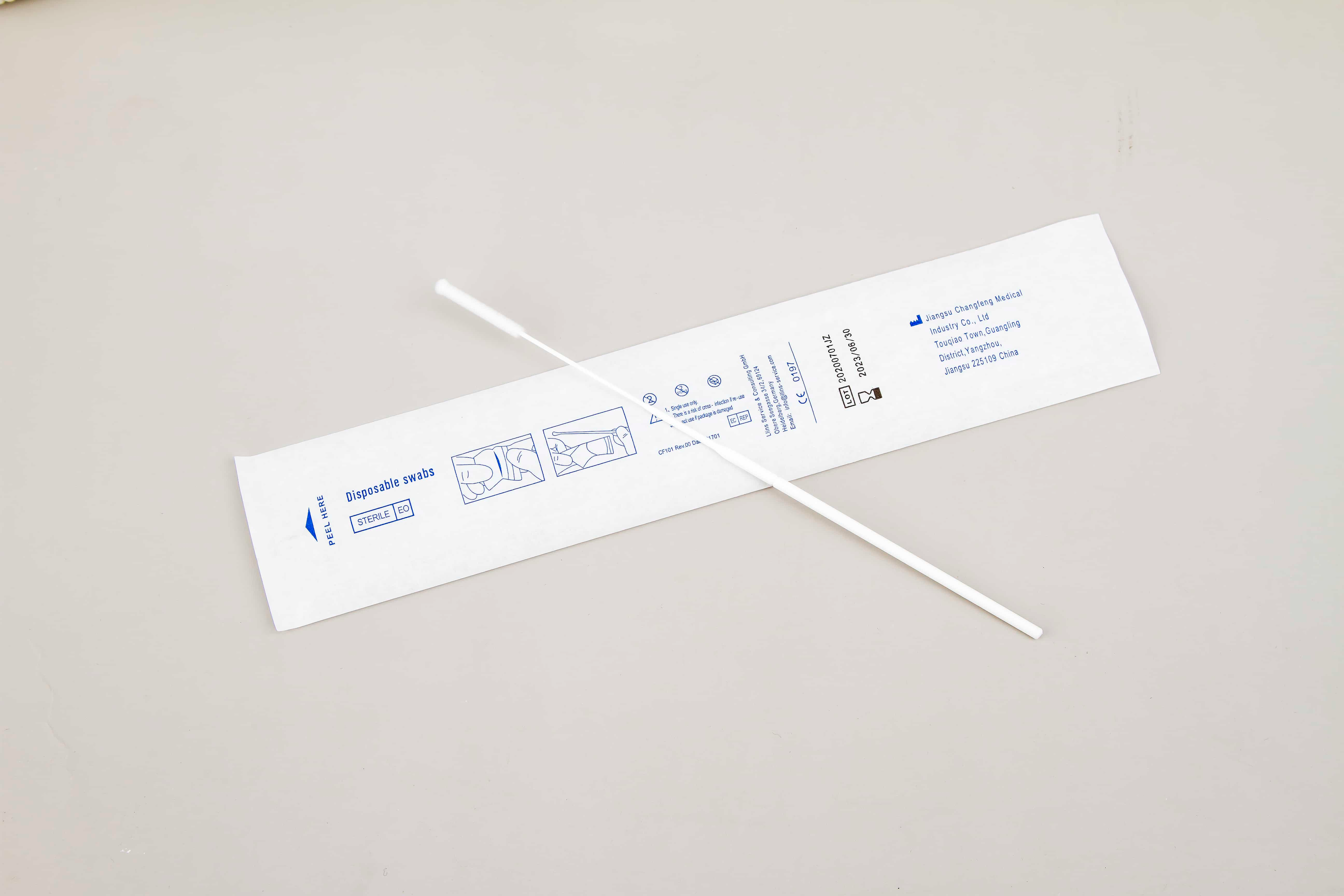
ဆေးဘက်ဆိုင်ရာ အမှန်အတိုင်းအတာကျသည့် ပွတ်သပ်စက္ကူများ၏ တိကျသော ထုတ်လုပ်မှု
စွမ်းအားကောင်းသော ဖျက်ဆီးရေးနည်းလမ်းများ- EtO နှင့် ဂမ္မာဓာတ်ရောင်ခြည်
ကျန်းမာရေးအတွက် စူပါဗိုင်းများ၏ ဘေးကင်းမှုနှင့် ထိရောက်မှုကို သေချာစေရန်အတွက် ဆေးဘက်ဆိုင်ရာအတွက် အသုံးပြုသည့် စူပါဗိုင်းများကို ဖျက်သိမ်းရာတွင် Ethylene Oxide (EtO) နှင့် Gamma Radiation တို့သည် အဓိကနည်းလမ်းနှစ်မျိုးဖြစ်ပါသည်။ EtO ဖျက်သိမ်းရေးသည် ရှုပ်ထွေးသော မိုက်ခရိုအော်ဂနစ် ဖွဲ့စည်းပုံများကို ဖြတ်သန်းဝင်ရောက်နိုင်သော စွမ်းရည်ကြောင့် ဆေးဘက်ဆိုင်ရာထုတ်ကုန်များကို နက်နဲစွာ ဖျက်သိမ်းရာတွင် အလွန်ထိရောက်ပါသည်။ သို့ရာတွင် ၎င်းသည် ဓာတုအမှုန့်အမှုန်များကို ကျန်ရစ်စေပြီး အပြည့်အဝ ဖယ်ရှားမထားပါက အန္တရာယ်များကို ဖြစ်ပေါ်စေနိုင်ပါသည်။ အတွင်းပိုင်း၊ Gamma Radiation သည် ပိုမိုမြန်ဆန်သော ဖျက်သိမ်းရေးလုပ်ငန်းစဉ်ကို ပေးဆောင်ပြီး အမှုန့်အမှုန်များကို မကျန်ရစ်ဘဲ မိုက်ခရိုအော်ဂနစ်များကို ဖျက်သိမ်းပေးသော်လည်း ဓာတ်ရောင်ခြည်ထိတွေ့မှုကြောင့် ပစ္စည်းများ၏ ဂုဏ်သတ္တိများကို သက်ရောက်နိုင်ပါသည်။
ဆေးရုံမှာ ကူးစက်မှုကိုတားဆီးရေးနှင့်ပတ်သက်သည့် ဂျာနယ်တွင် ဖော်ပြထားသည့် လေ့လာမှုများအရ အပ်ချုပ်ပြောင်းနှင့် ဂမ္မာဓာတ်ရေဒီယိုသည် ဘက်တီးရီယားပျက်စီးမှုကို ဖြစ်စေသော်လည်း ၎င်းတို့၏ ပျက်စီးမှုဖြစ်စဉ်မှာ ကွဲပြားသည်ဟု ဖော်ပြထားပါသည်။ အပ်ချုပ်ပြောင်းသည် DNA ဖွဲ့စည်းပုံကို ဖျက်စီးခြင်းဖြင့် ဘက်တီးရီယားပျက်စီးမှုကို ဖြစ်စေသော်လည်း ဂမ္မာဓာတ်ရေဒီယိုသည် ဆဲလ်အတွင်းဖြစ်စဉ်များကို တားဆီးခြင်းဖြင့် ဘက်တီးရီယားပျက်စီးမှုကို ဖြစ်စေပြီး အမှုန့်အမှားများ ကျန်ရစ်ခြင်းမရှိပေ။ ဤနည်းလမ်းများအတွက် လိုအပ်သည့် စည်းမျဉ်းများသည် FDA နှင့် WHO တို့က သတ်မှတ်ထားသည့် စံနှုန်းများနှင့် ကိုက်ညီမှုရှိစေရန် လုံခြုံရေးဆိုင်ရာ စိစစ်အတည်ပြုမှုများ ပြုလုပ်ရန် လိုအပ်ပါသည်။ လူနာများ၏ လုံခြုံရေးနှင့် ထုတ်ကုန်၏ ထိရောက်မှုကို သေချာစေရန်အတွက် ဤလုပ်ငန်းစဉ်များသည် အရေးကြီးပါသည်။
ကော်တွန်ချောမွေ့ထုတ်လုပ်မှုတွင် အရည်အသွေးထိန်းချုပ်မှု
စုပ်ယူသည့် ထုတ်လုပ်မှုအတွက် အရည်အသွေးစစ်ဆေးရေးလုပ်ငန်းစဉ်များတွင် အော်တိုမေးရှင်းမှုမှာ ပို၍အရေးကြီးလာပါသည်။ အဆင့်မြှင့်တင်ခြင်းနှင့် လူသားမှားယွင်းမှုများကိုလျော့နည်းစေခြင်းတို့ကြောင့် ဖြစ်ပါသည်။ မျက်စိစနစ်နှင့် AI အယ်လဂိုရီသမ်များကဲ့သို့သော အော်တိုမေးတစ်စနစ်များသည် စုပ်ယူသည့် ထုတ်ကုန်တိုင်းသည် တင်းကျပ်သော အရည်အသွေးစံနှုန်းများနှင့် ကိုက်ညီမှုရှိစေရန် အဓိကအခန်းကဏ္ဍမှပါဝင်ပါသည်။ ထုတ်လုပ်ရေးလုပ်ငန်းစဉ်များကို အမြဲစောင့်ကြည့်ခြင်းနှင့် အချိန်နှင့်တပြေးညီ တုံ့ပြန်မှုများပေးခြင်းအားဖြင့် ထုတ်ကုန်များ၏ တစ်ညီတည်းဖြစ်မှုကို ထိန်းသိမ်းပေးခြင်းနှင့် အမှားအယွင်းများကိုကာကွယ်ပေးခြင်းတို့အား ဤနည်းပညာများက ကူညီပေးပါသည်။
စိတ်ကူးမှသည့် အလိုအလျောက်ဖြစ်စေရန် ထိရောက်မှု၏ ဥပမာတစ်ခုကို swab ထုတ်လုပ်ရေးစက်ရုံများတွင် တွေ့မြင်နိုင်ပါသည်။ အရည်အသွေးစစ်ဆေးမှုများကို အလိုအလျောက် အကောင်အထည်ဖော်ခြင်းသည် ချို့ယွင်းမှုများကို သက်သာစေပြီး ထုတ်ကုန်ဘေးကင်းရေးကို တိုးတက်စေပါသည်။ ဉာဏ်ရည်တက်ကြွသော နည်းပညာများကို အသုံးပြုခြင်းဖြင့် ထုတ်လုပ်သူများသည် ကိုက်ညီမှုကို ယုံကြည်စွာ လိုက်နာနိုင်ပါသည်။ ဆေးဝါးထုတ်ကုန်များ ထုတ်လုပ်ရာတွင် မြင့်မားသော လိုအပ်ချက်များကို ဖြည့်ဆည်းပေးရန် စည်းမျဉ်းများ။ အလိုအလျောက်စနစ်သည် ထိရောက်မှုကို မြှင့်တင်ပေးသည့်အပြင် swab များ၏ ယုံကြည်စိတ်ချရမှုနှင့် ဘေးကင်းရေးကို ထိန်းသိမ်းရာတွင် အရေးကြီးသည်။ ဆေးစစ်ခြင်းအတွက် အသုံးပြုသည့် လူနာများ၏ ကျန်းမာရေးကို ထိခိုက်မှုမရှိဘဲ ရည်ရွယ်သည့်အတိုင်း လုပ်ဆောင်နိုင်သည်။
Swab ဒီဇိုင်းတွင် ပစ္စည်းအသစ်များ
နမူနာကောက်ယူမှုကို တိုးတက်စေရန် ဓာတုအညှောင်းများ
စုတ်ပိုးဒီဇိုင်းတွင် သဘာဝမဟုတ်သော အညှောက်များ အသုံးပြုခြင်းသည် အထူးသဖြင့် နမူနာကောက်ယူမှု ထိရောက်ဆဲလ်တွင် အဓိက အားသာချက်များ ပေးစွမ်းပါသည်။ သဘာဝမဟုတ်သော အညှောက်များကို နမူနာအမျိုးအစားများစွာကို ဖမ်းယူရန် ဖန်တီးထားပြီး စမ်းသပ်မှု ပလက်ဖောင်းများအလိုက် ရောဂါရှာဖွေမှု တိကျမှုကို တိုးတက်စေပါသည်။ ဥပမာအားဖြင့် ကော့တန် သို့မဟုတ် ရေယွန်းထက် သဘာဝမဟုတ်သော အညှောက်များသည် စုပ်ယူမှု ပိုမိုကောင်းမွန်ပါသည်။ ဤသည်များကို တိကျသော တိုင်းတာမှုများ အရေးကြီးသည့် ပတ်ဝန်းကျင်များတွင် အသုံးပြုရန် သင့်တော်ပါသည်။ နာမည်ကြီး အဖွဲ့အစည်းများမှ သုတေသနများအရ သဘာဝမဟုတ်သော အညှောက်များဖြင့် ပြုလုပ်ထားသော စုတ်ပိုးများသည် ဇီဝနမူနာများကို ဖမ်းယူရာတွင် တိကျမှု ပိုမိုမြင့်မားကာ စမ်းသပ်မှု ယုံကြည်စိတ်ချမှု နှင့် ရလဒ်များကို သိသာစွာ တိုးတက်စေပါသည်။ ထိုကဲ့သို့သော တီထွင်ဖန်တီးမှု ပစ္စည်းများကို အသုံးပြုခြင်းဖြင့် ထုတ်လုပ်သူများသည် တင်းကျပ်သော စမ်းသပ်မှု လိုအပ်ချက်များကို ဖြည့်ဆည်းပေးကာ ကျန်းမာရေးစောင့်ရှောက်မှု ပရော်ဖက်ရှင်နယ်များအတွက် ယုံကြည်စိတ်ချရသော ကိရိယာများ ပေးဆောင်နိုင်ပါသည်။
Saliva Collection Tube Systems နှင့် ကိုက်ညီမှု
စားပွဲတင် အသုတ်များသည် သွားဖုံစုဆောင်းသည့်ပြွန်စနစ်များနှင့် ကိုက်ညီမှုရှိကြောင်း သေချာစေခြင်းသည် နမူနာအရည်အသွေးကို ထိန်းသိမ်းပေးခြင်းနှင့် အသုံးပြုသူအတွက် အဆင်ပြေမှုကို သေချာစေရန် အဓိကအချက်ဖြစ်ပါသည်။ နမူနာများ ထိခိုက်ပျက်စီးခြင်း သို့မဟုတ် ဆုံးရှုံးမှုမဖြစ်စေရန်အတွက် ဒီဇိုင်းကို ပြေပြစ်စွာ ပေါင်းစည်းနိုင်ရန် လိုအပ်ပါသည်။ ဤစနစ်များနှင့် ကိုက်ညီသော စားပွဲတင် အသုတ်များကို ဖန်တီးခြင်းသည် အသုံးပြုရလွယ်ကူမှုကိုသာမက နမူနာအရည်အသွေးကို ထိန်းသိမ်းပေးခြင်းကိုလည်း သေချာစေပါသည်- မှန်ကန်သော ရောဂါရှာဖွေခြင်းတွင် အရေးကြီးသော အချက်တစ်ခုဖြစ်ပါသည်။ ဥပမာ - ဖလောက်စ်ဝဲ (flocked swabs) ကဲ့သို့သော အထူးဒီဇိုင်းများကို သွားဖုံစုဆောင်းသည့် လူကြိုက်များသော စနစ်များနှင့် အောင်မြင်စွာ ပေါင်းစည်းခဲ့ပြီး နမူနာကို ထိန်းသိမ်းထားနိုင်မှုနှင့် အသုံးပြုရလွယ်ကူမှုကို ပြသခဲ့ပါသည်။ စားပွဲတင် အသုတ်များသည် သွားဖုံစုဆောင်းရန် ရည်ရွယ်ထားပါက ရောဂါရှာဖွေမှုရလဒ်များကို ထိခိုက်မှုမရှိစေရန် သေချာစေရန် စံချိန်စံညွှန်းများနှင့် ကိုက်ညီမှုရှိရန် လိုအပ်သောကြောင့် စည်းကမ်းချက်များကိုလည်း ထည့်သွင်းစဉ်းစားရပါမည်။ ထုတ်လုပ်သူများသည် စီးပွားရေးလုပ်ငန်းဆိုင်ရာ စည်းကမ်းချက်များကို လိုက်နာရန် ထိရောက်သော စမ်းသပ်မှုများနှင့် စာရွက်စာတမ်းများကို ပံ့ပိုးပေးခြင်းဖြင့် ကိုက်ညီမှုရှိကြောင်း သက်သေပြရပါမည်။
စားပွဲတင် အသုတ်များ၏ ယုံကြည်စိတ်ချရမှုကို သေချာစေသည့် အသိအမှတ်ပြုလက်မှတ်များ
FDA ကိုက်ညီမှုနှင့် ISO 13485 လိုက်နာမှု
ဆေးဘက်ဆိုင်ရာ အသုံးပြုမှုအတွက် အရည်အသွေး လိုအပ်ချက်များနှင့် ကိုက်ညီမှုကို သေချာစေရန် FDA လက်မှတ်ရရှိရန် လုပ်ငန်းစဉ်များကို စနစ်ကျစွာ စစ်ဆေးပါသည်။ ထုတ်လုပ်မှုလုပ်ငန်းစဉ်များကို စုံစမ်းစစ်ဆေးခြင်းဖြင့် ဆေးဘက်ဆိုင်ရာ အသုံးပြုမှုအတွက် အန္တရာယ်ကင်းရှင်းမှုကို သေချာစေပါသည်။ FDA မှ ဆေးဘက်ဆိုင်ရာ အသုံးပြုမှုအတွက် လိုအပ်သော စံနှုန်းများနှင့် ကိုက်ညီမှုရှိစေရန် ပစ္စည်းများ၏ ဖွဲ့စည်းပုံနှင့် ပိုးစင်စစ်ခြင်းလုပ်ငန်းစဉ်များကို စိတ်ပိုင်းဖြတ်ပါသည်။ ISO 13485 စံနှုန်းများသည် ဆေးဘက်ဆိုင်ရာ ကိရိယာများအတွက် အရည်အသွေး တစ်သမတ်တည်းရှိစေရန် အဓိက အခန်းကဏ္ဍမှ ပါဝင်ပါသည်။ ဆေးဘက်ဆိုင်ရာ ဝန်ဆောင်မှုများအတွက် လိုအပ်သော အရည်အသွေး စီမံခန့်ခွဲမှုစနစ်များကို ထောက်ပံ့ပေးသည့်အားဖြင့် အရည်အသွေး အာမခံရေးကို သေချာစေပါသည်။ FDA နှင့် ISO 13485 လက်မှတ်ရရှိထားမှုများသည် စျေးကွက်အတွက် အကျိုးကျေးဇူးများစွာ ရရှိစေပါသည်။ ထိုသို့သော လက်မှတ်များသည် ထုတ်လုပ်သူများ၏ အမှတ်တရနှင့် ယုံကြည်စိတ်ချမှုကို မြှင့်တင်ပေးပြီး ဆေးဘက်ဆိုင်ရာ ကျွမ်းကျင်သူများနှင့် အဖွဲ့အစည်းများ၏ အမြင်တွင် ယုံကြည်စိတ်ချရမှုကို တိုးတက်စေပါသည်။
ကမ္ဘာတစ်ဝန်းရှိ ကျန်းမာရေးစံနှုန်းများအတွက် CE အမှတ်အသား
CE အမှတ်အသား လုပ်ငန်းစဉ်သည် ဥရောပဈေးကွက်များသို့ ဝင်ရောက်ရန် ရည်ရွယ်သော ဆေးဘက်ဆိုင်ရာ စက်ပစ္စည်း ထုတ်လုပ်သူများအတွက် အရေးကြီးပါသည်။ ဤထောက်ခံချက်သည် ထုတ်ကုန်များသည် ဥရောပသမဂ္ဂ၏ ကျန်းမာရေး၊ ဘေးကင်းရေးနှင့် ပတ်ဝန်းကျင် ကာကွယ်ရေး စံနှုန်းများနှင့် ကိုက်ညီကြောင်း ဖော်ပြသည်။ CE အမှတ်အသားရရှိရန်၊ ထုတ်လုပ်သူသည် ထုတ်ကုန်အကဲဖြတ်ခြင်းနှင့် ကိုက်ညီမှုစမ်းသပ်ခြင်းအပါအဝင် စေ့စေ့စပ်စပ်အကဲဖြတ်ခြင်းကို ခံယူရမည်ဖြစ်ပြီး၊ swabs ကဲ့သို့သော ကိရိယာများသည် တင်းကျပ်သော စည်းမျဉ်းစည်းကမ်းများကို လိုက်နာကြောင်း သေချာစေရမည်။ CE အမှတ်အသားဖြင့် ထုတ်လုပ်သူများသည် ၎င်းတို့၏ ထုတ်ကုန်များသည် ကမ္ဘာလုံးဆိုင်ရာ စံချိန်စံညွှန်းများနှင့် ကိုက်ညီကြောင်း အာမခံပြီး ကျယ်ကျယ်ပြန့်ပြန့် လက်ခံမှုနှင့် အသုံးပြုမှုကို လွယ်ကူချောမွေ့စေပါသည်။ CE အမှတ်အသားပါသော ထုတ်လုပ်သူအတွက် စျေးကွက်အကျိုးသက်ရောက်မှုအထောက်အထားများတွင် ပို့ကုန်အခွင့်အလမ်းများ တိုးမြင့်လာခြင်းနှင့် အရည်အသွေးမြင့် ပရိုတိုကောများကို လိုက်နာခြင်းအတွက် ခိုင်မာသောဂုဏ်သတင်း ပါဝင်သည်။ ဤအသိအမှတ်ပြုလက်မှတ်သည် ဥရောပတွင် တံခါးဖွင့်ပေးရုံသာမက ၎င်းကိုကိုယ်စားပြုသည့် တင်းကျပ်သောစံချိန်စံညွှန်းများကို အသိအမှတ်ပြုသည့် နိုင်ငံတကာဈေးကွက်များတွင် ထုတ်ကုန်များကို ထောက်ခံပေးပါသည်။
စွမ်းဆောင်ရည်စမ်းသပ်မှုစည်းမျဉ်းများ
စုပ်ယူနိုင်မှုစွမ်းရည်အတည်ပြုနည်းလမ်းများ
စုဆောင်းထားတဲ့ နမူနာတွေရဲ့ တည်ကြည်မှုနဲ့ စိတ်ချရမှုကို အာမခံဖို့ swab တွေရဲ့ စုပ်ယူမှုစွမ်းရည်ကို အတည်ပြုခြင်းဟာ အရေးပါပါတယ်။ အတည်ပြုမှုအတွက် စက်မှုလုပ်ငန်း စံနှုန်းစနစ်များတွင် မကြာခဏဆိုသလို ဓာတ်ခွဲခန်းအခြေခံ စမ်းသပ်မှုများ ပါဝင်သည်၊ ဥပမာ စုပ်ယူနိုင်သည့် အရည်ပမာဏကို တိုင်းတာသည့် gravimetric ဆန်းစစ်မှုနှင့် အရည်များ ထိန်းသိမ်းခြင်းနှင့် ထုတ်လွှတ်ခြင်းများကို အကဲဖြတ်သည့် spectrophotometric ဆန်းစစ်မှုတို့ဖြစ်သည်။ ဒီစမ်းသပ်ရေး ပရိုတိုကောတွေဟာ ဆေးဘက်ဆိုင်ရာ အသုံးအဆောင် အမျိုးမျိုးမှာ အသုံးပြုတဲ့ swab တွေအတွက် တစ်သမတ်တည်း လုပ်ဆောင်မှု စံချိန်တင်မှတ်တမ်းတွေ သတ်မှတ်ဖို့ ကူညီပေးပါတယ်။ စက်မှုလုပ်ငန်း လမ်းညွှန်ချက်များအရ swab များသည် တိကျသော ရောဂါစစ်ဆေးမှု ရလဒ်များအတွက် အရေးပါသော နမူနာ၏ တည်ကြည်မှုကို ယုံကြည်စိတ်ချစွာ ထိန်းသိမ်းနိုင်ရန်အတွက် တင်းကျပ်သော စုပ်ယူမှု စမ်းသပ်မှုများသည် မရှိမဖြစ်လိုအပ်သည်။ ဒီစစ်ဆေးမှုတွေရဲ့ အရေးပါမှုကို သုတေသနက ပြသတာက မြင့်မားတဲ့ စုပ်ယူမှုစွမ်းရည်ဟာ နမူနာ ပြန်လည်ထုတ်ယူမှုနှုန်း တိုးတက်မှုနဲ့ ဆက်စပ်နေကာ ဆေးပညာရှင်တွေရဲ့ ရောဂါရှာဖွေရေး အရည်အချင်းတွေကို မြှင့်တင်ပေးတာပါ။
အချိန်နှင့်တပြေးညီ ညစ်ညမ်းမှု စောင့်ကြည့်ရေး စနစ်များ
တိကျမှုရှိသော ဆေးဘက်ဆိုင်ရာ ပွတ်သပ်ခြင်းများ၏ အနှစ်သာရကို ထိန်းသိမ်းရာတွင် ကူးစက်မှုကို စောင့်ကြည့်ထိန်းချုပ်မှုစနစ်များသည် အဓိက အခန်းကဏ္ဍမှ ပါဝင်ပါသည်။ ဤစနစ်များသည် ကူးစက်မှုများကို တစ်ပြိုင်နက်တည်း ဖယ်ရှားရန် ဖြေရှင်းချက်များကို ချက်ချင်း လုပ်ဆောင်နိုင်စေရန် အောပ်တစ်ခဲဆန်ဆာများနှင့် စက်ရုပ်သင်ယူမှု အယ်လဂိုရစ်များကဲ့သို့ နည်းပညာများကို အသုံးပြုပါသည်။ ဥပမာအားဖြင့် ဤစောင့်ကြည့်ထိန်းချုပ်မှုစနစ်များဖြင့် ကျန်းမာရေးစောင့်ရှောက်မှု အဆောက်အဦများသည် အရည်အသွေးထိန်းချုပ်မှုလုပ်ငန်းစဉ်များတွင် သိသာထင်ရှားသော တိုးတက်မှုများကို အစီရင်ခံပြီး ကူးစက်မှုကြောင့် မှားယွင်းသော အပေါင်းအကျိုးများကို နည်းပါးစေပါသည်။ အခြေခံလေ့လာမှုမှ အချက်အလက်များအရ တစ်ပြိုင်နက်တည်း စောင့်ကြည့်ထိန်းချုပ်မှုစနစ်များ ကျင့်သုံးခြင်းဖြင့် ကူးစက်မှုကြောင့် ဖြစ်သော အမှားများကို ၆၀% အထိ လျော့နည်းစေနိုင်ပြီး ဆေးဘက်ဆိုင်ရာ ရောဂါရှာဖွေမှုများ၏ ယုံကြည်စိတ်ချရမှုကို တိုးတက်စေပါသည်။ ဆေးဘက်ဆိုင်ရာ ပွတ်သပ်ခြင်းများ၏ သန့်ရှင်းရေးနှင့် တိကျမှုကို သေချာစေရန် ဤရှေ့တိုးဝင်စောင့်ကြည့်မှု ချဉ်းကပ်မှုကို လူနာများအား ကျန်းမာရေးစောင့်ရှောက်မှု စံနှုန်းများကို တိုးတက်စေရန် ဆိုင်းငံ့နေသော ဆေးရုံများနှင့် ဆေးခန်းများက ပိုမို၍ ကျင့်သုံးလျက်ရှိပါသည်။
အကြောင်းအရာများ
- ဆေးဘက်ဆိုင်ရာ အမှန်အတိုင်းအတာကျသည့် ပွတ်သပ်စက္ကူများ၏ တိကျသော ထုတ်လုပ်မှု
- စွမ်းအားကောင်းသော ဖျက်ဆီးရေးနည်းလမ်းများ- EtO နှင့် ဂမ္မာဓာတ်ရောင်ခြည်
- ကော်တွန်ချောမွေ့ထုတ်လုပ်မှုတွင် အရည်အသွေးထိန်းချုပ်မှု
- Swab ဒီဇိုင်းတွင် ပစ္စည်းအသစ်များ
- စားပွဲတင် အသုတ်များ၏ ယုံကြည်စိတ်ချရမှုကို သေချာစေသည့် အသိအမှတ်ပြုလက်မှတ်များ
- စွမ်းဆောင်ရည်စမ်းသပ်မှုစည်းမျဉ်းများ